Faster Time to Market for Your Medical Device or Application
Are you looking to bring a medical device or software to the market with full confidence in its quality and compliance? As an ISO 13485 certified partner, we specialize in the development of medical software that is not only reliable but also aligned with US quality and regulatory requirements.
Expert Software Development and Solutions for Medical Devices
In the highly regulated US healthcare landscape, the path from a medical software idea to market entry demands compliance with rigorous standards, stringent regulations, and unwavering attention to detail.
At Atostek, we specialize in medical device software development. We pride ourselves on our deep understanding of FDA regulations and our commitment to compliance with US quality and safety standards.
Our team specializes in delivering advanced software services and solutions that not only align with but surpass the rigorous requirements of the medical device industry. We ensure that our clients’ medical devices achieve market clearance efficiently and without compromise to patient safety.

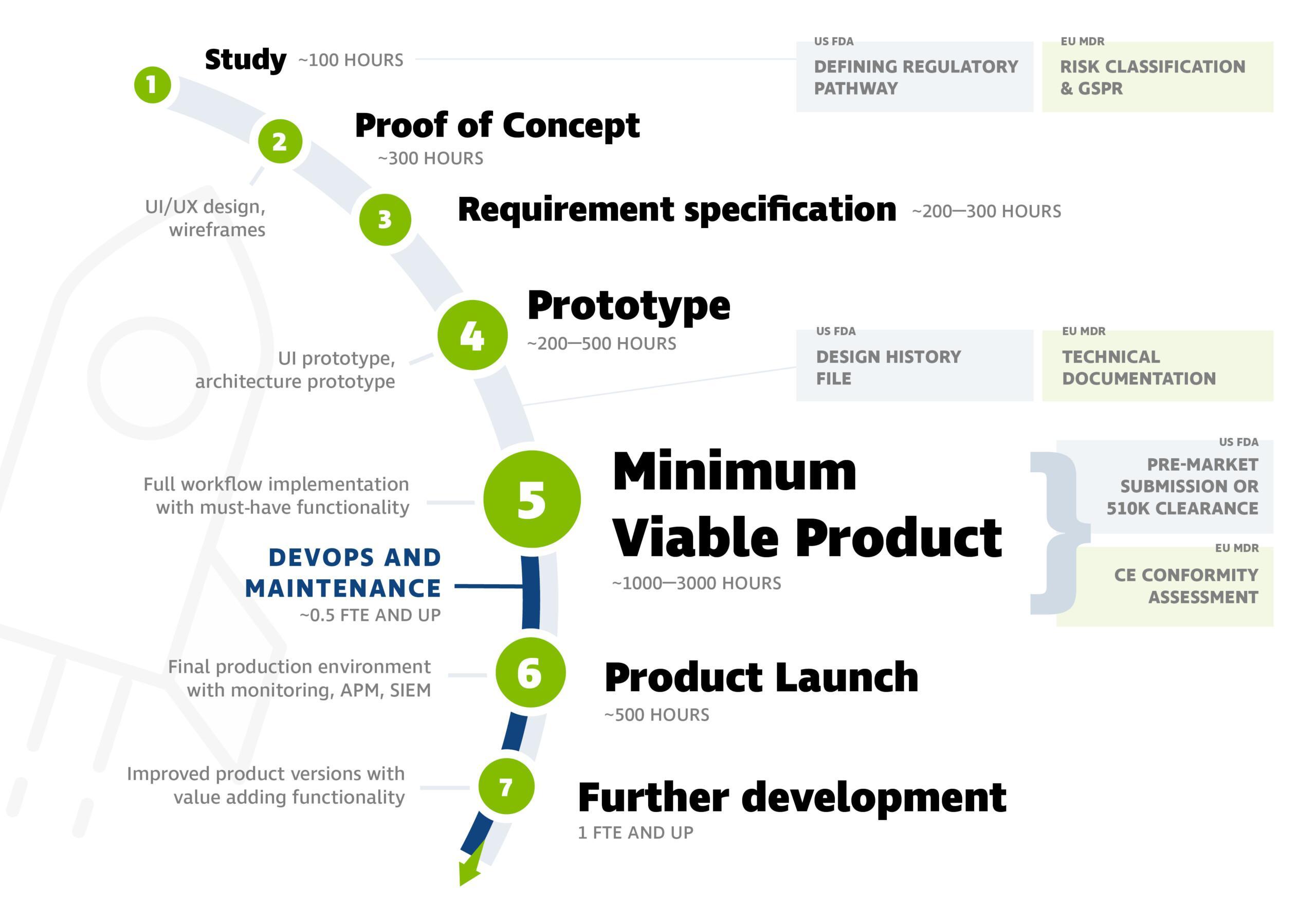
Software Development Process
The software development process for medical devices typically progresses according to the following stages. You can see an example of workload estimates in the picture below.
The picture includes steps from the beginning, but the project can also be initiated at later stages. Often, the product is ready for the market at stage 5 (Minimum Viable Product).
PET ERP – Enterprise Resource Planning for PET Centers
Is it difficult to schedule academic research and diagnostic PET studies at the same time? Are you searching for a solution to efficiently schedule a large number of PET scanners and tracers? With PET ERP, you can streamline your PET centre’s operational processes, data traceability and tracking.
LET OUR CUSTOMERS TELL

MYNDSPAN
“Through our collaborative efforts with Atostek, we have succeeded in creating a new kind of solution for monitoring the human brain. The cooperation has gone smoothly and the solid expertise of Atostek’s medical software has played a decisive role throughout the project.”
Janne Huhtala, CEO
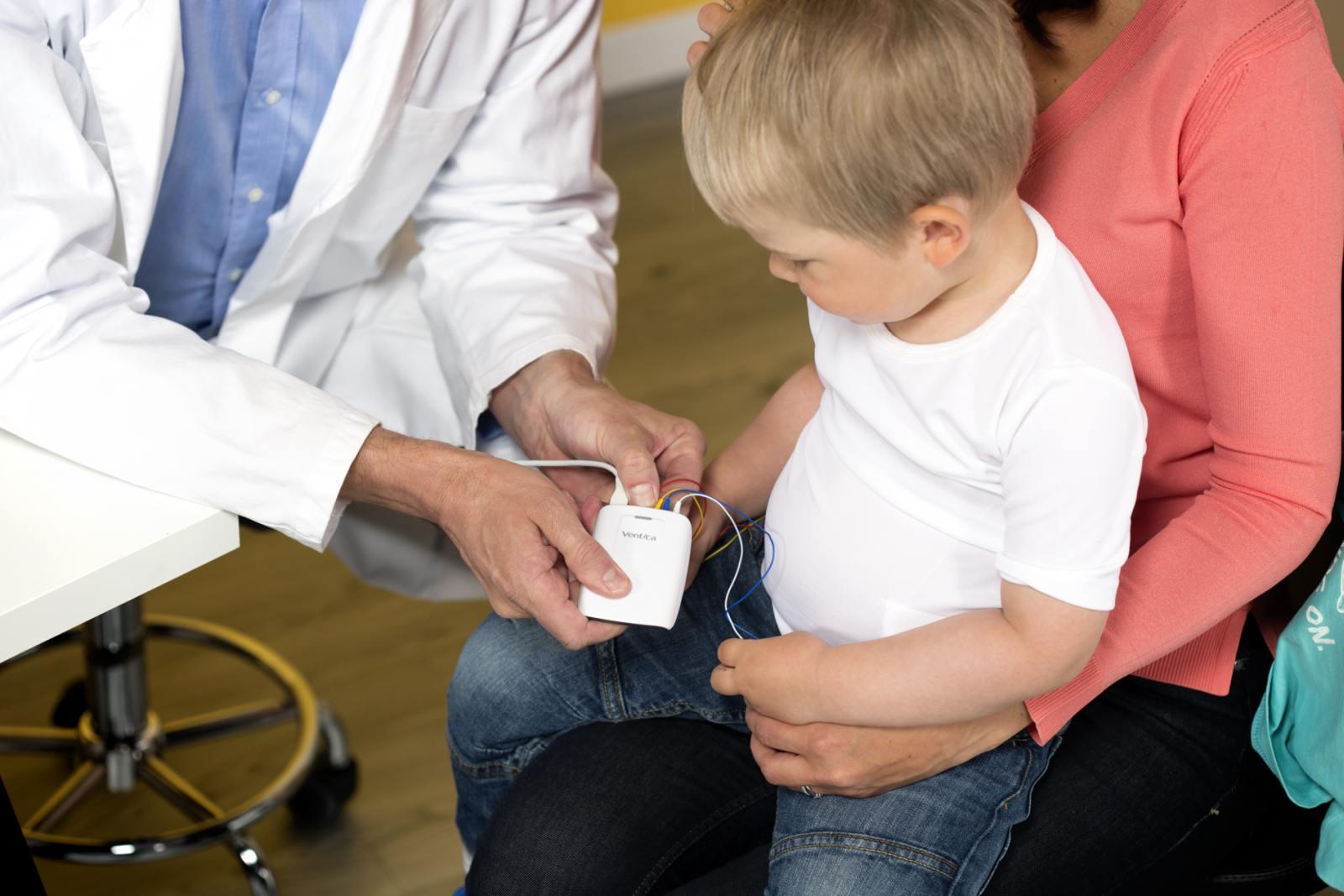
REVENIO
Atostek developed software and productised the Matlab-based calculation method for Revenio’s Ventica asthma diagnosis product. Work included developing web-based service for initializing, performing and reporting the measurements and developing the communication method between USB-connected measurement device and web service.