Software Development and Solutions for Medical Devices
The software development of a medical device is carefully regulated, from the design stage all the way until the software has been approved. Knowledge of the industry’s quality requirements and carefully planned and executed actions play an important role throughout the process of ensuring human health and safety.
We specialise in cutting-edge software services and solutions that meet the stringent requirements of medical devices.

Our Solutions
Flexbot
Our specification-driven framework empowers you to develop robust software for medical devices and robotics, driving the future of automated healthcare. With Flexbot, you can accelerate product development and save on costs while considering the strict safety requirements of medical devices and software.
FHIRWORX
Looking to enhance the operations of your healthcare organization? Do the integrations of information systems seem too complicated? Our FHIRWORX service helps you integrate with patient information systems, implement artificial intelligence and analytics, and gather all the essential data you need in one place.
Software Development Process
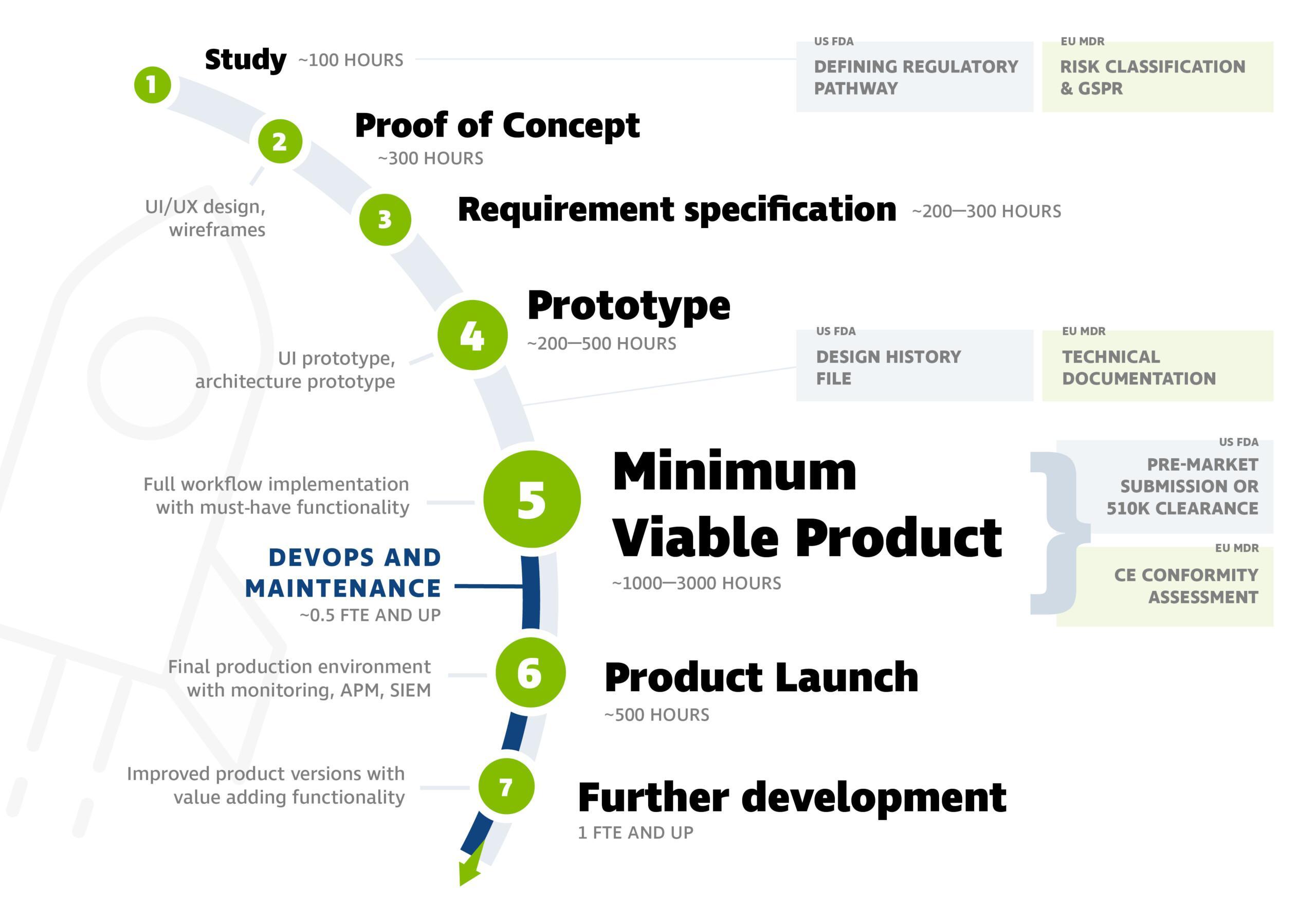
The software development process for medical devices typically progresses according to the following stages. You can see an example of workload estimates in the picture below.
The picture includes steps from the beginning, but the project can also be initiated at later stages. Often, the product is ready for the market at stage 5 (Minimum Viable Product).
Our Guarantee
Atostek offers services for the software development of medical devices. We have been awarded the ISO 13485 certificate for medical devices and software, which applies to development of medical software for clients. We also guarantee that we operate in accordance with the applicable regulatory requirements.

Regulations
- EU Medical Device Regulation (EU MDR) – CE marking
- US FDA Medical Device Regulation
Standards
- ISO 13485 – Quality Management
- IEC 62304 – Software Lifecycle
- ISO 14971 – Risk Management
- IEC 62366 – Usability
Technology: Integrations and Platform Solutions
A seamless flow of information is vital in the medical field. We make sure that the systems used are mutually compatible, and we create the necessary solutions. We have expertise in communication standards and technologies such as HL7 CDA R2, FHIR, DICOM, and XDS.
We create scalable production environments using various means, such as public cloud platforms. We have implemented applications and solutions based on public cloud platforms for many of our clients. The platforms we use the most are Microsoft Azure and Amazon AWS.
Read More
Medical Software Testing According to ISO 13485 – 5 Things to Consider
In our white paper, we tell you more about medical software testing according to ISO 13485. We highlight five things that should be taken into account when planning and implementing effective and standard-compliant testing of medical software. Download our white paper and read more!


How to Design Medical Device Usability
Are you planning to design and market a medical device, either a physical one or a SaMD (Software as a Medical Device)? According to regulatory requirements, considering usability is mandatory. You have to apply the IEC 62366-1 standard in the design and evaluation process – the primary purpose of usability is to consider patient safety. Download our white paper and read more!
Juho Leppämäki (Europe)
Sales, Medical Devices
Quality Manager
juho.leppamaki@atostek.com
+358 45 113 8883
Juhani Perhonen (USA)
Chief Business Developer
juhani.perhonen@atostek.com
+1 914 530 4069 (USA)
+358 45 7834 5589